Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mine, Tatsuya*; Mihara, Morihiro;
JNC TN8430 2000-009, 35 Pages, 2000/07
In the geological disposal system of TRU wastes, nitrogen generation by denitrifying bacteria could provide significant impact on the assessment of this system, because nitrate contained in process concentrated liquid waste might be electron acceptor for denitrifying bacteria. In this study, the activities and tolerance of denitrifying bacteria under disposal condition were investigated. pseudomonas denitrificans as denitrifying bacteria was used. The results showed that Pseudomonas denitrificans had activity under reducing condition, but under high pH condition (PH 9.5), the activity of Pseudomonas denitrificans was not detected. It is possible that the activity of Pseudomonas denitrificans would be low under disposal condition.
9.5), the activity of Pseudomonas denitrificans was not detected. It is possible that the activity of Pseudomonas denitrificans would be low under disposal condition.
Kato, Hiroshige*; Sato, Mitsuyoshi*; Owada, Hitoshi*; Mihara, Morihiro;
JNC TN8430 2000-008, 53 Pages, 2000/05
Cementitious materials will be used in TRU waste disposal repository. In such cases, it is considered that the migration of alkaline leachates from cementitious materials, so called high pH plume, will cause dissolution of rock and precipitation of secondary minerals. In addition, the high pH plume will move along the flow of groundwater, so it is predicted that rock formation and components of high pH groundwater vary with time and space. However, time and spatial dependence of the variations of secondary minerals and groundwater components has not been clarified. In order to acquire the data of variations of secondary minerals and groundwater components, we carried out the rock alteration experiments with column method. The crushed granodiorite was filled into 4 meters length column (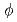 3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80
3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80 C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
Savage, D.*; Lemke, K.*; Sasamoto, Hiroshi; Shibata, Masahiro; Arthur, R. C,*; Yui, Mikazu
JNC TN8400 2000-004, 30 Pages, 2000/01
Modeling approaches that have been proposed for cement-water system are reviewed in this report, and relevant supporting thsrmodynamic data are compiled. The thermodynamic data include standard molal thermodynamic properties of minerals and related compounds comprising cements, and equilibrium constants for associated hydrolysis reactions. Similar data for minerals that are stable in hyperalkaline geologic environments (e.g., zeolites) are also included because these minerals could be formed as hyperalkaline fluids emanating from cementitious matelials in a repository for radioactive wastes interact with the surrounding host rock. Standard molal properties (i.e., standard molal Gibbs free energies and enthalpies of formation and standard molal entropies), and/or equilibrium constants for associated hydrolysis reactions, are included for. (1)cement minerals and related compounds (Reardon, 1992; Glasser et al., 1999) (2)calcium-silicate hydrate minerals (Sarkar et al., 1982), and (3)zeolites (calorimetric and estimated values from various sources) All these data are accepted at face value, and it is therefore cautioned that the data, considered as a whole, may not be internally consistent. It is also important to note that the accuracy of these data have not been evaluated in the present study. Several models appropriate for cement-water systems have been proposed in recent years. Most are similar in the sense that they represent empirical fits to laboratory data for the CSH gel-water system, and therefore not thermodynamically defensible. An alternative modeling approach based on thermodynamic principles of solid-solution behavior appropriate for CSH gel has recently been proposed, however. It is reviewed in the present study, and evaluated in relation to experimental results obtained by JNC on cement-water interactions. The solid-solution model is based upon a thermodynamically- and structually-justifiable description of CSH gel in terms of a non-ideal ...
Sato, Tsutomu*; Oda, Chie
no journal, ,
Bentonite buffer and cement fluid interaction has been a key research issue in long-term safety assessment of radioactive waste disposal. The dissolution rate of smectite, the main constituent mineral of bentonite, has therefore been investigated under hyperalkaline conditions. A dissolution rate equation of smectite has been derived considering the effect of the Gibbs free energy of reaction and is applicable to pH = 7-13 and t = 25-80 C. Reactive-transport model analyses to elucidate the consequences of coupled changes in the porewater chemistry, mineralogy and, ultimately, the mass transport properties of the bentonite buffer were conducted using the smectite dissolution rate. Except in the close proximity of the cement interface, it was found that regardless of the choice of secondary minerals, the effective diffusion coefficient and hydraulic conductivity remained largely unchanged after 100,000 years.
C. Reactive-transport model analyses to elucidate the consequences of coupled changes in the porewater chemistry, mineralogy and, ultimately, the mass transport properties of the bentonite buffer were conducted using the smectite dissolution rate. Except in the close proximity of the cement interface, it was found that regardless of the choice of secondary minerals, the effective diffusion coefficient and hydraulic conductivity remained largely unchanged after 100,000 years.